Biopharmaceutical drug development
Biologics, or biopharmaceutical drugs, are promising treatments with low side effects for several diseases including cancer, immunological disorder and infectious disease. The high cost of biologics treatment causing the low drug accessibility and affordability. Each year Thailand imports approximately 500-1,000 million Baht of these products from many foreign sources.
CBRD is focusing on translating fundamental scientific research to solve national and global health issues using multidisciplinary cutting-edge technology. Since the pandemic of bird flu in 2004 in Thailand, Her Royal Highness Princess Chulabhorn realized that Thailand was in an urgent need of national health security for self-reliance on therapeutics. Biologics or biological products consist of variety classes of bioactive agents including vaccines, blood products, tissue therapy, cell therapy, gene therapy, and recombinant proteins. CBRD main focus is currently on development of recombinant proteins and biologics with potential to be therapeutics, vaccines, diagnostics, and research reagents. Current focus is primarily but not limited to monoclonal antibody, virus like particle, and recombinant peptide hormone.
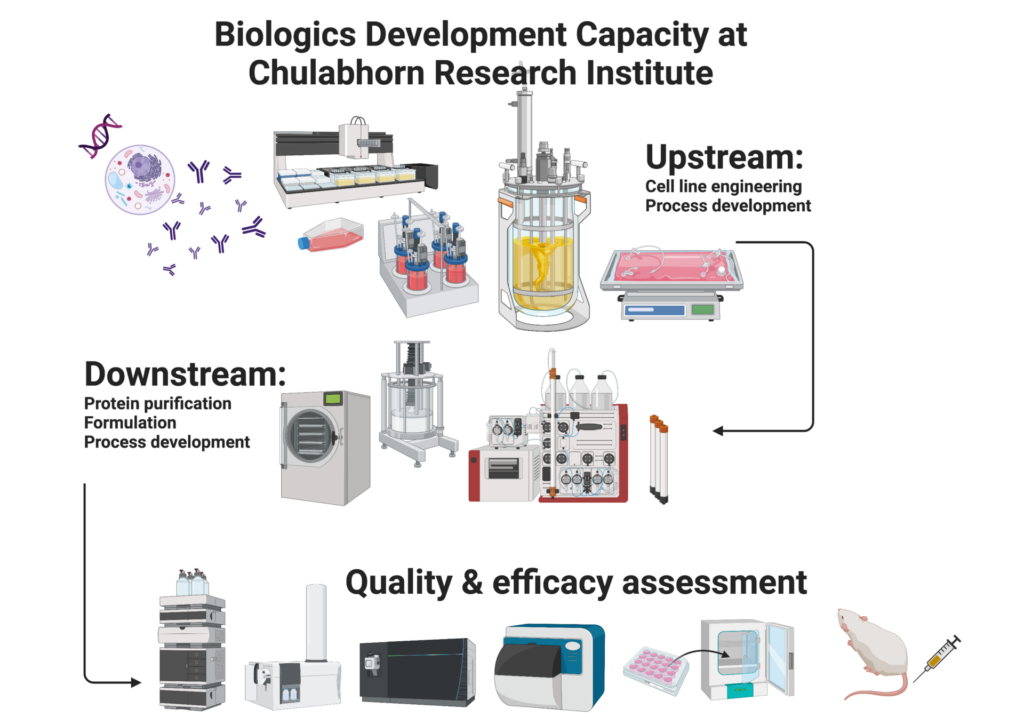
To ensure the seamless transition and preparation of product pipeline, CRI has integrated all necessary steps required for biologics drug development, which are lead screening, lead identification, lead optimization, characterization assay establishment, efficacy demonstration, process development, and proof of concept study. With the lighting speed of technology shift nowadays, CRI has been invested in state-of-the-art technology for product development and quality assessment tools to ensure the forefront research quality. Due to the translation goal, the research’s key considerations are the compliance to latest regulatory guideline, the simple and scalable process, and the continuous improvement of both technology and human resources.
For product development, a variety of approaches have been implemented such as hybridoma technology, biosimilars and in silico product design. Series of recombinant protein production technique are applied including different molecular techniques, variety of expression systems (bacterial, insect, and mammalian cell), and various purification techniques (normal flow filtration, tangential flow filtration, ultracentrifugation, and chromatography). Cell line development is performed in accordance to international guideline to ensure the compliance for regulatory registration. Upstream and downstream process development at s small scale are conducted at laboratory scale.
Product quality is the determination of product safety and efficacy. Product quality assay developed at CBRD including both lot release testing and deep characterization. The wide arrays of assays were developed to orthogonally cover different aspects of product such as identity, purity/impurity, physical properties, strength and efficacy. A variety of more than 100 characterization methods ranging from basic to sophisticated techniques are utilized such as cell-based assays, high throughput or high-resolution kinetic binding interaction assays, chromatographic-based techniques, diverse mass spectrometry techniques, spectrophotometry-based assays, and capillary electrophoresis-based assays, etc.





