Laboratory of Pharmacology


At present, it is well recognized that environmental exposure is linked to the pathophysiology of a number of chronic diseases including cancers, diabetes, cardiovascular diseases, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s disease. We have a mission to understand the basic mechanisms of diseases, pathogenesis, and influences of environmental factors on neurodegeneration, diabetes, cancer, and cardiovascular-associated diseases.
Particular interests are paid to cholinergic muscarinic receptors (MRs), and glycogen synthase kinase-3 (GSK3) signaling pathways in which both involve diverse array of cellular functions ranging from cell proliferation, glucose metabolism and cell death. Moreover, both MRs and GSK3 influence inflammation, a process that plays critical roles in development of many chronic diseases and is triggered by environmental factors like air pollutants and pesticides. Additionally, dysregulation of GSK3 has been implicated in insulin resistance type II diabetes and Alzheimer’s disease (AD).
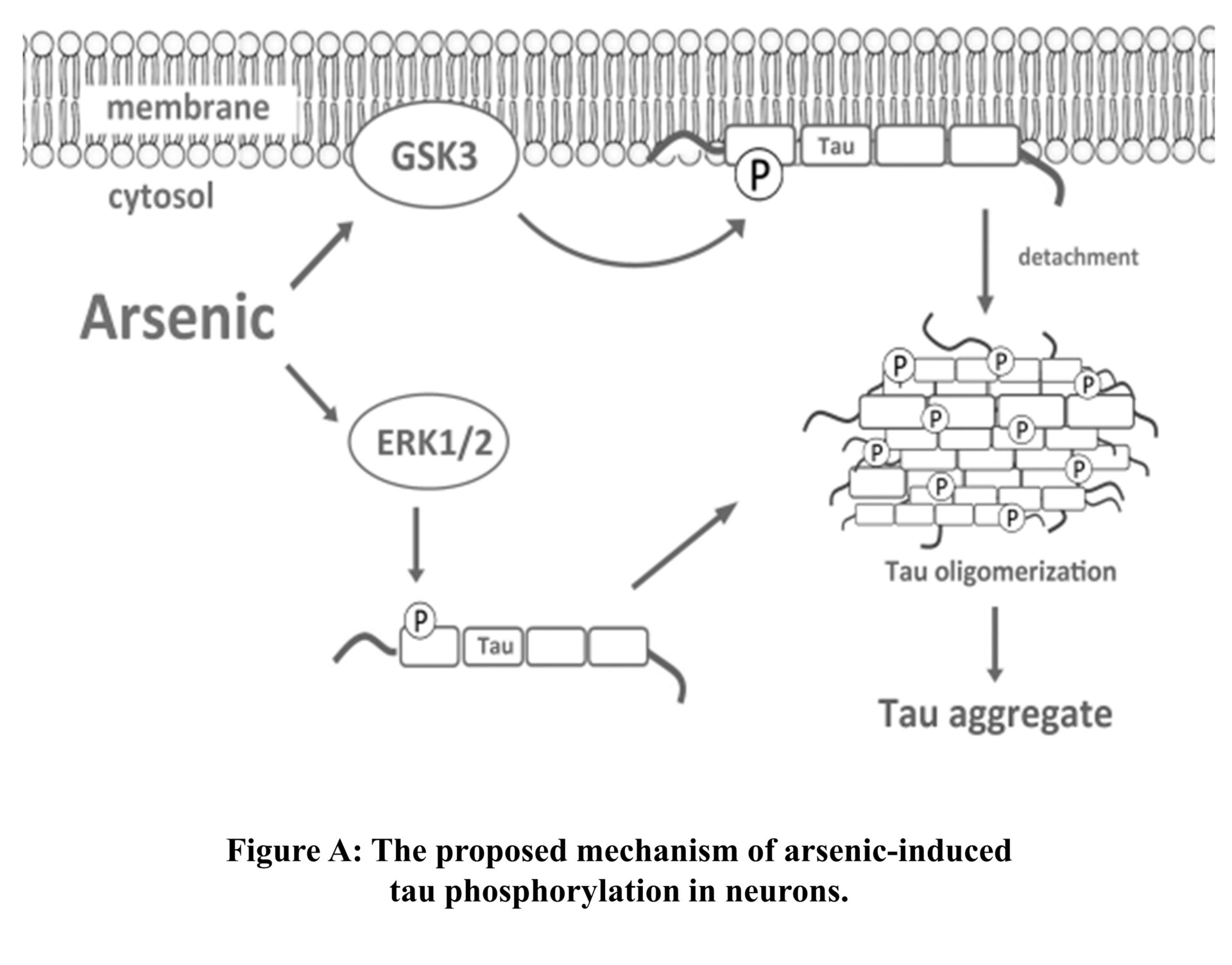

Nowadays, the interconnection of insulin resistance and AD has been clearly established. As arsenic has been reported to cause both insulin signalling impairment and AD–like effects such as memory deficit thus our laboratory is interested in finding the contribution of insulin signaling and AD as well as other neurodegenerations such as Parkinson’s disease. It is ongoing study to examine arsenic affects insulin signaling in the brain using cell lines and animal model, and whether brain insulin signaling alteration is associated with its neurodegeneration action. We hope to gain more understanding regarding how arsenic causes neurodegenerative effects as well as the roles of insulin/insulin signaling in brain function.
In addition, the impact of environmental pollutants that affect estrogen signaling is also an area of interest. Study on the effect of heavy metals such as cadmium and arsenic which possess estrogenic and/or antiestrogenic properties on biology of cancer is in progress. The study emphasizes both the genomic and non-genomic estrogen signaling pathways. The ability of these metals to alter estrogen signalings may be significant for their tumorigenicity and toxicity effects. Furthermore, the xenoestrogenic activity of herbicides and industrial compounds have been investigated.
Glyphosate is one of the most common used herbicides in Thailand which has a high risk for contamination in the environment. Our previous in vitro study has shown that the low and environmentally relevant concentrations of glyphosate could stimulate breast cancer cell growth via a mechanism involving the estrogen receptors. In addition, we also conducted research to investigate the effects of glyphosate, on the estrogen and the other signaling pathways involved in the induction and progression of cholangiocarcinoma (CCA) cell lines. Our study revealed that glyphosate induced growth of CCA cells with the involvement of ERα estrogen receptor and ERK1/2 signaling pathway. These results explain, at least in part, molecular mechanism of how glyphosate act on the estrogen signaling pathway in inducing proliferation of cancer cells.
Although the epidemiological study for assessing exposure and outcome associations is significant, the mechanistic understanding for many of the disease manifestations is somewhat limited. Chronic diseases such as cancers have a long latency period from exposure to disease, and often have multiple mechanisms of action. Oxidative stress is one such mechanism, cancer is postulated to resulting from mutations induced through DNA damage caused by
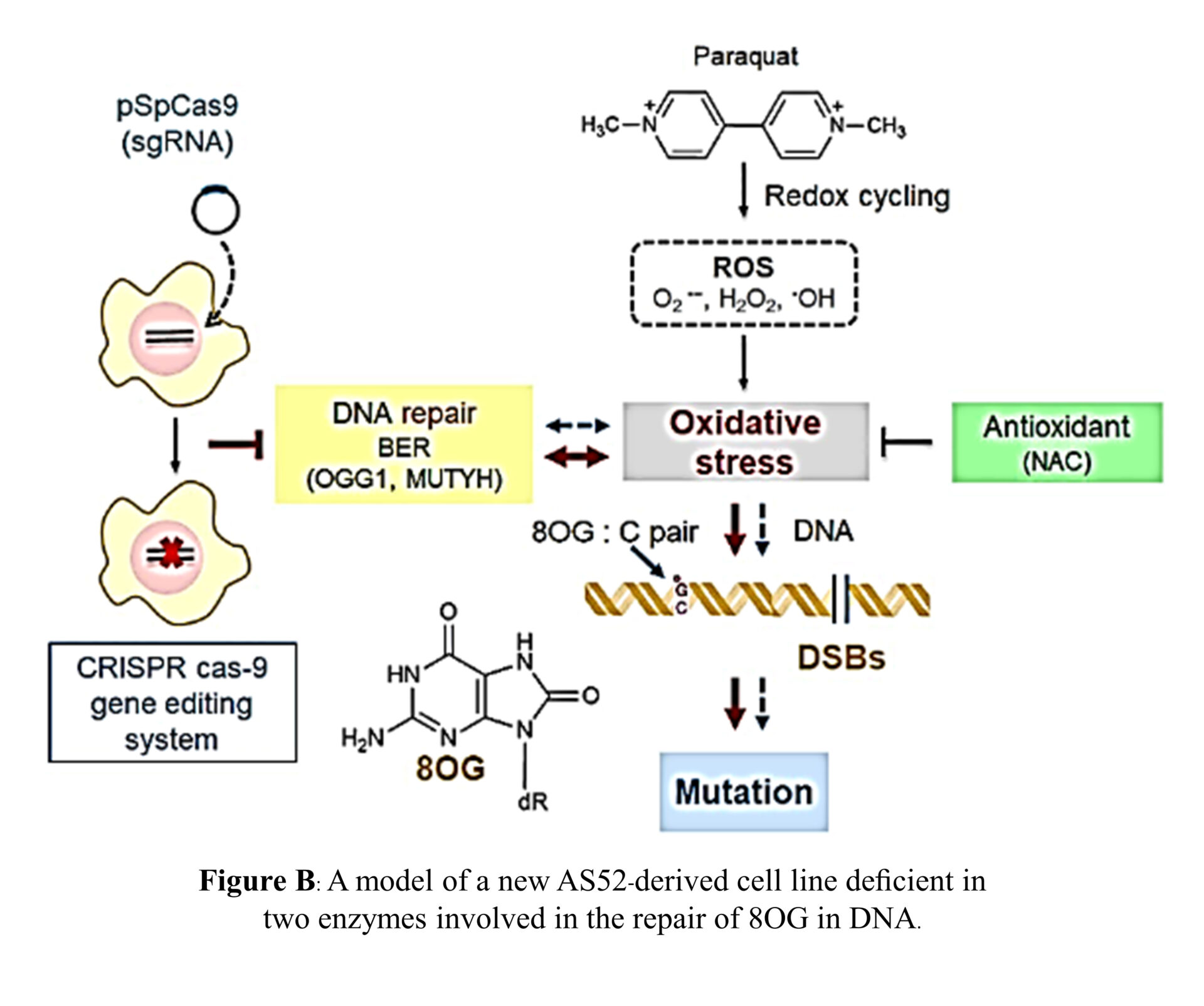
reactive oxygen species (ROS). Paraquat (PQ): a broad-spectrum herbicide, for example, is thought to exert its effects through pathways that involve oxidative stress, which can result from generation of ROS. Our previous studies demonstrated that PQ-induced genomic instability through generation of oxidative-stress induced the formation of mutagenic and oxidative DNA lesions in a new constructed hamster cell culture system (AS52-derived cells) defective for 8-oxoguanine DNA glycosylase and MutY homolog, the two key enzymes involved in oxidative DNA damage repair. This cell line has been employed as a sensitive tool model for studying mutagenic effects of low-level environmental agents or weak mutagens that induce oxidative stress.