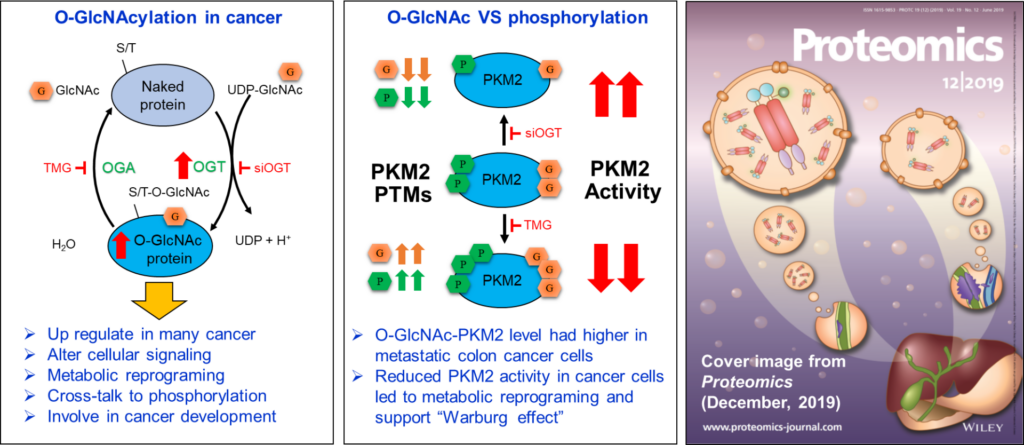
Post-translational Modifications in Cancer
Many proteins are chemically modified after synthesis. These Post-Translational Modifications (PTMs), such as phosphorylation and O-GlcNAcylation, play an important role in regulating metabolic processes. Our studies show that the levels of O-GlcNAcylation and O-GlcNAc transferase (OGT), an enzyme catalyzing the addition of a single GlcNAc residue to target proteins, were up-regulated in breast and colon cancer tissues and cell lines. Using mass spectrometry, many O-GlcNAc-modified proteins were identified in these cancers, for example pyruvate kinase M2 (PKM2). O-GlcNAcylation of PKM2 affects its phosphorylation as well as its activity, thus indicating the crosstalk between these two PTMs. We also demonstrated that OGT knockdown led to a significant reduction of colony formation in breast and colon cell lines, suggesting it plays vital roles in tumor progression.
Phosphorylation is another regulatory mechanism, but identification of phosphoproteins can sometimes be difficult due to possible digestion by phosphatase enzyme. We have therefore isolated exosomes, extracellular vesicles (EVs) of 30-100 nm size, from the conditioned media of isogenic cholangiocarcinoma cell lines with different metastatic potential. More than 40 phosphoproteins were identified with significant change in phosphorylation level. Heat Shock Protein 90 was confirmed as showing differential phosphorylation in relation to tumor invasiveness, so aberrant phosphorylation of exosomal proteins may be useful for development of a metastatic cancer biomarker. Indeed, since EVs contain many functional biomolecules, such as oncoproteins, active miRNAs and signaling molecules, we plan to perform a more in-depth study using a multi-disciplinary approach to understand the molecular function of EVs, especially in relation to cancer.