Process Chemistry for Preparing Active Pharmaceutical Ingredients (APIs)
For some selected compounds already prepared on a lab-scale, the corresponding procedures appropriate for their scale-up to the pre-pilot (kilogram) levels have been carefully executed; this is highly critical to reflect the practicability of the developed protocols and serves as a bridge to further translate them from the laboratory to the industry. Selection of a synthetic route must accommodate methods of purification which, towards the industrial applications, involve either crystallization for solid or distillation for liquid with minimal, if any, column chromatography. Other equipment necessary for the separation of organic- and aqueous-soluble layers is also vital. Moreover, appropriate analytical instruments such as high-performance liquid chromatography (HPLC) to follow the progress of the reaction must accommodate the real-time analysis.


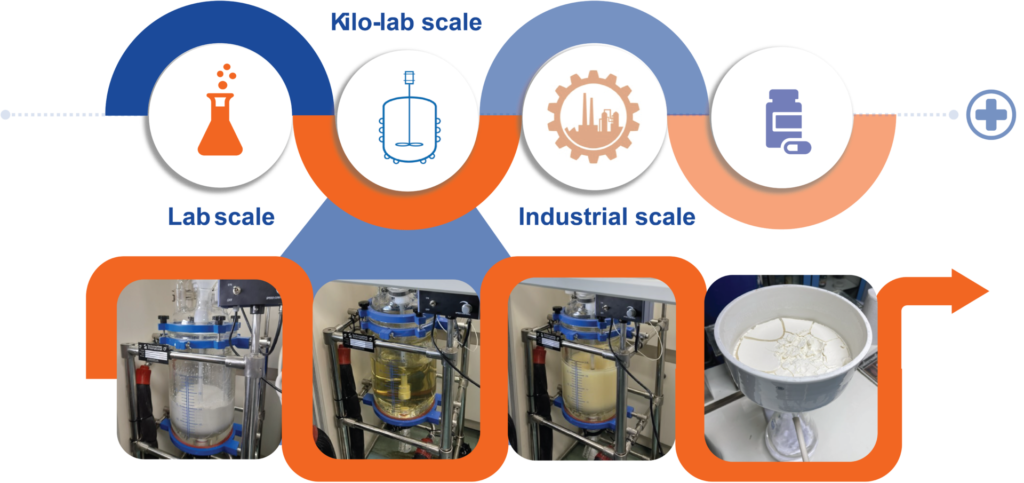

During the COVID-19 pandemic, our laboratory has successfully upscaled the preparation of the anti-viral drug molnupiravir starting from cytidine. Two synthetic routes—one using only chemicals and the other using immobilized lipase—have been successfully executed to furnish the desired product in only 2-3 steps in comparable overall yields on the kilogram scales. Using enzyme as a biocatalyst also conforms to the Thai government policy on the bio-circular-green economy (BCG) for sustainable development. This is an important step forward to establish the requisite process chemistry in Thailand for producing APIs on larger scales.





